Application Guide
Pre-configured Customized Kits with Low Volume H-Cells to Conduct Advanced Level Carbon Dioxide Reduction Electrocatalysis Studies and New Materials Research
IP-HC5:Fully Equipped H-Cell Kit
Contents
Introduction
The H-Cells Design and Fundamentals for Electrochemical Carbon Dioxide Conversion
BASi Electrochemical-H-Cell Options
Next Generation Potentiostat / Galvanostat Customized for Electrocatalysis Studies: Nexus Engineered for Electrochemical Excellence
PSTrace Software Platform
Reference Articles
Introduction
In 2022, U.S. greenhouse gas emissions totaled 6,343 million metric tons (14.0 trillion pounds) of carbon dioxide equivalents. Emissions increased from 2020 to 2022 by 5.7 percent, driven by an increase in carbon dioxide emissions from fossil fuel combustion due to economic activity rebounding after the height of the pandemic.
The increase in the emission of greenhouse gas such as CO2 has been proven as one of the main reasons for global warming. Hence the research and development in the areas of technologies to reduce the carbon dioxide emission in air have attracted extensive attention. Over the past 30 years, various technologies have been developed to address this climate change issue. Among them, the electrochemical or photo-electrochemical methods have been considered as a promising candidate for commercial level CO2 reduction to produce various useful chemicals, such as formic acid, acetone, methane, and more that could be further utilized as industrial feedstock. However, the efficiency and selectivity of the catalyst materials used on the electrodes during the electrochemical process are not satisfactory. The efficient catalysts play a very crucial for the electrochemical conversion of CO2, which need further deeper studies to improve the overall catalytic performance.

Approach to the extraction of energy sources and raw materials by reducing CO 2 .
J. Roth. & T.J. Schmidt. Energy storage in research: Research into new technologies in Switzerland. The Bulletin Electrosuisse 3. 2016
The H-Cells Design and Fundamentals for Electrochemical Carbon Dioxide Conversion
Owing to their customization ability to desired types of electrodes and geometrical designs, their economic feasibility, and ease of operation, H-Cells set-ups are the most commercially available lab scale electrochemical cells for Carbon Dioxide Reduction Studies in modern world. A typical H-Cell is a two-compartment electrochemical cell used for membrane testing, Hydrogen permeation, or any other experiment where two separate electrode chambers are required. Each chamber is usually equipped with three glass joints for gas-purging or adding electrodes or sensors. The two compartments are filled with needed volume of electrolytes in which the working electrode (usually coated with desired catalyst) and reference electrodes occupy the cathodic compartment, while the counter electrode is placed in the anodic compartment. Both compartments are separated by an ion-exchange membrane at the H-joint. Thus, any byproduct generated at the counter electrode does not affect the working electrode's behavior and vice versa. The design allows a gas-tight setup. CO2 gas is fed into the cathodic chamber wherein gas and liquid products can be collected after the reduction reaction. On the other hand, oxidation (oxygen evolution reaction with water) happens at the anodic chamber to maintain the charge balance by closing the circuit.
H-type cells are capable of screening different catalysts in a short span of time, making it easy to operate and cost effective. For example, a series of Sn catalytic electrodes were prepared using electro-deposition method with controlled current density at room temperature and applied to the electrochemical reduction of CO2 as shown below. The experimental results showed that the deposited Sn catalysts exhibited a crystal pattern of the (1 0 1) and (1 1 2) faces and the thickness of deposited layer varied with the deposition current density. The activity of these electrodes was determined in H-type and 3-elelctrode system, and the reduction product was quantitatively analyzed by ion chromatography. The optimized deposition current density for Sn fabrication was found to be 15 mA cm−2, on which the Faradaic efficiency of CO2 conversion was over 91% at −1.4 V vs. SCE.
 C. Zhao & J.Wang. Electrochemical reduction of CO2 to formate in aqueous solution using electro-deposited Sn catalysts.
C. Zhao & J.Wang. Electrochemical reduction of CO2 to formate in aqueous solution using electro-deposited Sn catalysts.Chemical Engineering Journal, 293, 2016, Pages 161-170. https://doi.org/10.1016/j.cej.2016.02.084
BASi Electrochemical-H-Cell Options
BASi Research Products provides IP-HC cell configurations (5-1000 mL volume) are a fully equipped with all the spares and accessories needed to be used in plug-n-play fashion. These are horizontally mounted and a dual compartment electrochemical H-Cell set-ups for a wide variety of studies that require a separate compartment for counter and / or reference electrodes. The construction is gas-tight having two separate chambers each with 25-1000 ml volume capacity (as needed) and each equipped with gas inlet and outlet option. This allows bubbling the solution and evacuating gases. Chambers can be separated with an ion-exchange membrane (Nafion 117 equivalent provided by BASi) or working electrode stainless steel specimen (for hydrogen permeation studies), so the electrochemical products appearing at working and counter electrode do not affect the opposite electrode. An L-shaped Lugging capillary can be provided for reference electrodes in case of hydrogen permeation tests. Upon request, each chamber can be temperature controlled using a glass tube that can be placed inside each chamber. Set-up is compatible with a power source meter or a typical Potentiostat / Galvanostat. We also provide Teflon or PTFE based H-cells in Sandwich shaped geometry with reduced distance between the reference electrode and membrane.
How to set-up BASi H-cell and Photo-EC H-Cell

|
Available Volumes: 5 mL, 10 mL, 25 mL, 50 mL, 100 mL, 250 mL, 500 mL, and 1000 mL H-Cell Accessories: IP-GC-50H-1P: A pair (Qt. 2) of thermal control glass tube coils for temperature control in each chamber of IP-HC50 and IP-PECHC50 cells. IP-HC-Lug-M-50: Lugging tube for Ref. Electrode near membrane in IP-PECHC50 or IP-HC50. IP-N117-Eq: Hydrogen permeation membrane Equivalent to N117 - 5/pack for H-CELLS and PEC H-CELLS. Thickness: 183 µm, Diameter: 35mm, Density: 360 g/m2 Recommended Optional Items: Reference Electrodes (Aqueous or Non-Aqueous), Working Electrode, Auxiliary (Counter) Electrodes , MF-2024: Working electrode (flat samples like ITO) holder. |
Next Generation Potentiostat / Galvanostat Customized for Electrocatalysis Studies
Nexus Engineered for Electrochemical Excellence
The Nexus uses the latest advancements in technology and offers perfectly accurate electrochemical results with ultra-low-noise. The instrument offers a broad array of electrochemical techniques and stores all measurements internally on a 32 GB backup enabling hyper-fast long measurements. The research software PSTrace makes using the Nexus a breeze. The scripting language Method SCRIPT gives a user full control when needed. Looking for a multi-channel instrument? Just stack multiple instruments on top of each other.
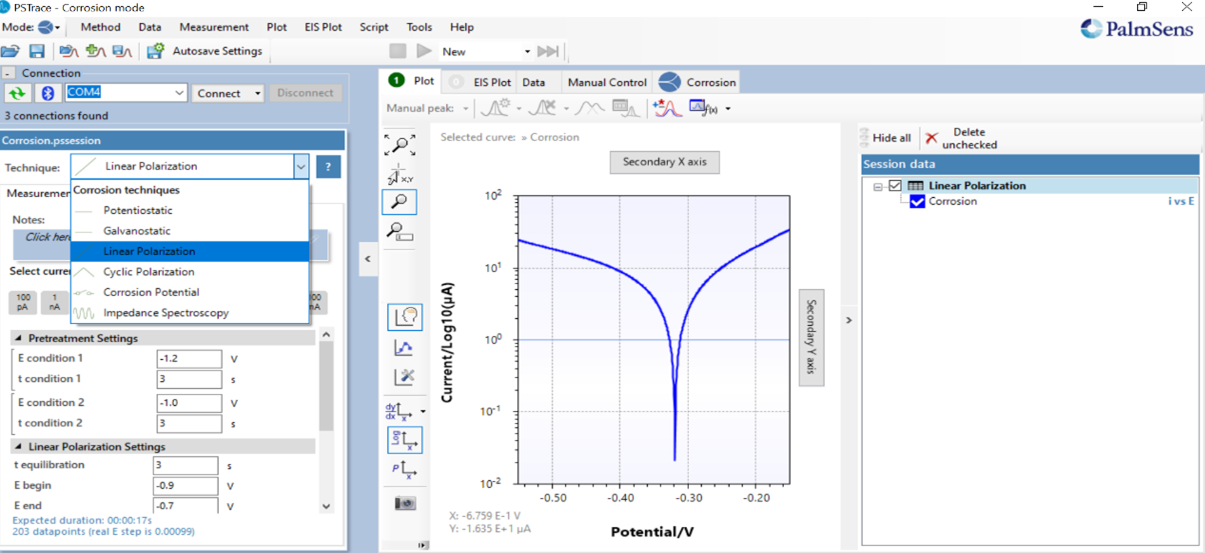
PSTrace Software Platform
The PalmSens4 Potentiostat also includes our complementary PSTrace Software Platform that is equipped with a sophisticated mode for Corrosion Analysis that provides more than 20 modern world corrosion analysis techniques ready-to-go for Tafel-slope based Corrosion Rate Studies, Open Circuit Voltage, Linear / Galvanostatic Polarization, Galvanostatic Cycling, Corrosion potential analysis, hydrogen permeation analysis in floating mode, and much more.

Reference Articles:
C.G. Armstrong et al. Exploring the electrochemistry of iron dithiolene and its potential for electrochemical homogeneous carbon dioxide reduction. ChemElectroChem 9, 2022, 202200610. https://doi.org/10.1002/celc.202200610
G. Neri et al. In situ study of the low overpotential “dimer pathway” for electrocatalytic carbon dioxide reduction by manganese carbonyl complexes. Phyical Chemistry Chemical Physics 21, 2019, 7389-7397. https://doi.org/10.1039/C9CP00504H
Posted March 28, 2025



